SESSION C
Radioactive Aerosols, Health Effects
275
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
276
IASTA-2010
C–O–1
A Study on Aerosol Charging in Generation Mechanism
Amit Kumar, V. Subramanian, R. Baskaran, J. Misra
and P. Chellapandi
Radiological Safety Division, Safety Group,
Kalpakkam-603 102, India
Introduction
In the aerosol studies on nuclear safety, the generation of aerosols are envisaged in following process viz. (i) mechanical de-fragmentation in mining and milling process, (ii) vapourisation and condensation route for the production of fuel and fission product aerosols in the case of reactor accident, (iii) combustion route for the production of sodium compound aerosols, (iv) incineration of low level radioactive wastes and (iv) HEPA filter bank testing by generating test aerosols by atomization. Understanding the mechanism of aerosol charging during generation of aerosols is important in aerosol science, particularly to understand the aerosol process like particle deposition, electrical migration, and sampling and transport etc. When we consider the above cases there exist diversity in aerosol materials and production, accordingly, the charge induction by the aerosols during generation would vary. In this study, aerosols are generated in three process viz. (i) combustion route (generating sodium compound aerosols), (ii) vaporization and condensation route (SrO2 aerosols) and (iii) atomization route (polystyrene latex aerosols) and induction of charges on these aerosol in the generation process is studied using Electrical Low Pressure Impactor and the results are presented.
Aerosol Generation
The studies are conducted in Aerosol Test Facility[1]. The facility is equipped for the generation of sodium aerosols by combustion route and SrO2 aerosols by vaporisiation and condensation route by using 20kW thermal plasma torch. The facility has an aerosol generator (M/s Grimm aerosol generator, GmbH, Germany) for the production of Polystyrene latex aerosols by atomization. The ELPI (M/s Dekati Ltd., Finland) is used for the measurement of charge-size distribution.
Sodium aerosol generation : About 5 g of sodium is heated in Argon environment upto 500°C. The sodium is melted and molten sodium is ignited by purging atmospheric air after letting the argon. The sodium combustion takes place and the sodium compound aerosols that are generated are bottled-up in aerosol chamber.
Generation of aerosols by plasma synthesis: A known weight of SrO2 powders (M/s ACROS Organics, USA) were palletized. Typical weight of the pellets were 5±0.2 g. The pellets were rigidly fixed in to the wire feeder tube and exposed to plasma flame. The pellet melts and the molten vapours after leaving the flame zone condense to produce aerosols, which are flown into the aerosol chamber.
277
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
Generation of polystyrene latex aerosols by atomization : Using Polystyrene latex particle (M/s Polyscience Inc., USA) of 1.3 m in the aerosol generator, (Model No:7.811, M/ s Grimm aerosol generator, GmbH, Germany) mono-dispersed aerosols are generated. Atomization is carried out in Ethyl Alcohol solution to which few drops of polystyrene latex particles are added. Alcohol vapours are made to evaporate by heating the transport line by using heating coil wounded over the line. The aerosols are made to suspend in a chamber where charge measurement are carried out.
Charge-size measurement: By using ELPI with corona charger off condition, the charges associated with aerosols were measured as charge-size distribution. Also with corona charger on condition number-size distribution were measured. By using charge-size distribution, number-size distribution, the flow rate of the instrument and by taking the elementary charge value, the average number of charges associated with the aerosols is determined.
Results and Discussions
Table 1 shows the average number of charges acquired by the aerosols having size nearly 1.29 m determined by ELPI. The ELPI measures the charge-size distribution for all its impaction size ranges (0.007-10 μm). From all the data, number of particles and the charge associated with 1.29 μm sized particles are taken for the comparison. It is to be noted here that, initial size distribution of sodium combustion aerosols at 50%RH is 1.0 m,[2], hence polystyrene latex particles of 1.3 m is used for atomization technique, while vapourisation and condensation route produce multi-model aerosols, of which, particles corresponding to 1.29 m is chosen for this study.
Table 1: Average number of charges associated with 1.29 m aerosols generated in various methods
| Generation method | Aerosol material | Average number |
| of charges abs<j> | ||
| Combustion | Sodium compound | 1.8 |
| Vaporisation and | SrO2 | 0.4 |
| condensation | ||
| Atomization | Polystyrène latex | 253 |
| particules | ||
Note : 1.29 m is geometric mid point obtained for the size range 1.021-1.655
In the case of combustion route, the particles are formed generally as a solid by condensation of sodium fumes. The condensation produces little or no charge on the aerosols but these aerosols acquire charges by Brownian collision with omnipresent air ions and attain Boltzmann equilibrium. In vaporization and condensation route, particle charging is significant as evaporation produce significant number of high mobility ions. Hence charge-associated with these aerosols also attain Boltzmann equilibrium. In the atomization process of polystyrene latex particles, it is observed that average-number of charges associated with these aerosols are maximum. Since polystyrene is a dielectric liquid associated with surface charges, during the atomization the charged surface gets disrupted hence the aerosols acquire charges by spray electrification. Fig. 1 shows the typical charge size distribution of sodium combustion aerosols and its number concentration.
278
IASTA-2010
Particle diameter ( m)
Figure 1. Average charge-size distribution of sodium combustion aerosols and its number concentration
Summary
Aerosol charging during generation is more in atomization of dielectric liquid. However there exists slight difference in the number of charges acquired by the aerosols generated through combustion and vapourisation-condensation process though they aerosols are formed by condensation.
References
1.“Aerosol charaterisation and measurement techniques towards SFR safety studies”, R. Baskaran, V. Subramanian, J. Misra, R. Indira, P. Chellapandi and BaldevRaj, ANIMMA-2009, International conference in Advancements in Nuclear Instrumentation, Measurement Method and their Application, June 7-10, 2009, Marseille, CEA, France.
2.“Initial size distribution of sodium combustion aerosols”, V. Subramanian and R. Baskaran, Nuclear Technology, Vol.160, p.308 (2007).
279
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
C–O–2
Deposition Velocity of Strontium Peroxide Aerosols and
Particle Morphology
J. Misra, N. Balajia, V. Subramanian and R. Baskaran
Radiological Safety Division, Safety Group
Indira Gandhi Centre for Atomic Research, Kalpakkam – 603 102. India a M.Tech Student, Bharathiar University, Coimbatore.
Introduction
In the event of Core Disruptive Accident (CDA) condition of Fast Reactor,The Reactor Containment Building (RCB) is bottled-up with large amount of coolant, fuel and fission product aerosols. To understand the aerosol behaviour in the RCB, some of the aerosol parameters like, particle size distribution, coagulation constant, sedimentation rate constant etc are to be determined. These parameters are used as an input to the codes (like HAARM, AEROSIM) to predict suspended mass concentration of aerosols from time to time, gravitational settling and wall plating. In the event of CDA, the expected mass concentration of aerosols inside RCB would be few tens of grams per cubic meter, hence the aerosol motion no longer exhibit as an individual particles and the drag force experienced would be sum of all the particles i.e as a cloud. The gravitational settling is considered to be as deposition velocity of a particle cloud rather than terminal settling velocity of individual particles. Also morphology of particles is an important factor for the determination of particle shape factor ‘ ’. It is to be noted that the aerosol formation in reactor accidents is essentially considered to be vaporization-condensation process, hence plasma torch method of generation of aerosols is adopted. Experiments are conducted in Aerosol Test Facility (ATF) by generating Strontium peroxideb aerosols upto a mass concentration of few g/m3 and deposition velocity is determined by using Turn Table Instrument (developed indigenously at IGCAR) and particle morphology is determined using SEM. The experimental results are presented in this paper.
Experimental
The experiments are carried out in Aerosol Test Facility (ATF), of Radiological Safety Division; IGCAR. ATF consists of an aerosol chamber of volume one cubic meter, a plasma torch for the production of fission products and fuel equivalent aerosols and a sodium combustion cell for the generation of sodium aerosols. It has many Aerosol Diagnostic equipments such as Filter paper sampler, Mastersizer, Low Pressure Impactor (LPI), Aerosol Spectrometer with dilutor, Sequential Mobility Particle Sizer (SMPS) and Electrical Low Pressure Impactor (ELPI). The diagnostic equipments are integrated into the aerosol chamber through various sampling ports which satisfies the Davis criteria to ensure negligible sampling error for still air sampling condition for the particles below
280
IASTA-2010
20ìm. The facility is equipped with a humidity controller, pressure transducer and thermocouples integrated into a Data Acquisition System.
A known weight of SrO2 powders (M/s ACROS Organics, USA) was palletized. Typical weight of the pellets was 5±0.2 g. The pellets were rigidly fixed in to the wire feeder tube and exposed to plasma flame. The pellet melts and the molten vapours after leaving the flame zone condense to produce aerosols, which are flown into the aerosol chamber. The aerosol characterization was carried out with filter paper sampler for the determination of mass concentration. The number-size distribution (measured by using SMPS) and the count median aerodynamic diameter are determined. The particles are made to deposit on the aluminum foil fixed in the collection plates of the Andersen Low Pressure Impactor and the morphology of the particles is analyzed through SEM. The turn table instrument and the optical microscope are utilized to determine the number flux, and it is used for determining the deposition velocity of the aerosols.
Results and Discussions
| Initial experimental runs were carried | ||||
| out for standardizing the strontium | ||||
| peroxide aerosol | mass concentration. | |||
| SrO2 pellets were exposed to 20kW | ||||
| plasma flame. | Typical count-size | |||
| distribution is shown in Fig.1, the count | ||||
| median aerodynamic diameter is found | ||||
| to be 230.35 nm and the standard | ||||
| deviation is calculated to be 1.63. The | ||||
| number concentration is determined to | ||||
| be 8.4x105 particles/cm3. | ||||
| Estimation of number flux : Turn Table | ||||
| instrument is used to measure the number | Figure 1. Number-size distribution of SrO2 | aerosols | ||
| / mass flux. | It | has a collection plate | ||
with 16 slots, on to which aerosols are made to deposit. A glass plate is mounted on each of the 16 slots and exposed to aerosols one at a time for known period of time. The number flux is obtained by counting the number of particles, deposited for the known interval of time in optical scan microscope (M/s Zeiss Opti Scan, Germany) and using the area of the substrate. The number flux is estimated as:
The number flux is found to be 93.91 cm-2s-1. The number flux obtained by turn table equipment for the first 30s and corresponding measurement of number concentration measured by SMPS for the same time is used to determine deposition velocity.
Estimation of Deposition Velocity :
Deposition velocity is defined as 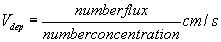
281
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
The typical value of deposition velocity is found to be 1.17x 10-4 cm.s-1. This deposition velocity is attributed to count median aerodynamic diameter of 230.35 nm (sg = 1.63). It is to be noted here that, aerosol sampling was carried out simultaneously. The study is repeated with 25 kW plasma power. The CMAD is determined as 143.3 nm (sg = 1.57), and corresponding number flux and deposition velocity are found to 98.43 cm-2s-1 and 1.12x 10-4 cm.s-1 respectively.
Particle Morphology : The particle morphology is studied for the particles collected in first experiment. The SEM analysis of the particles deposited on the collection plate of L4 (ECD 110 nm) and L5 (ECD 80 nm) of the Low Pressure Impactor is shown in the Fig. 2
(a) and 2 (b) respectively. The figures show the accumulation of particles as well as very fine individual spherical shaped particles. Interestingly cluster aggregates are not seen in the 100 nm range. Hence the plasma synthesis of nanometer sized SrO2 aerosols is spherical in nature and the shape factor ‘ ’ can be taken as 1. This is achieved by the torch parameters resulting in very high saturation ratio of the vapor and subsequent homogeneous nucleation of supersaturated vapor during the collection period.
| Figure | 2(a). SEM Images of SrO2 | aerosols with ECD | Figure 2(b). SEM Images of SrO2 aerosols with ECD |
| 110nm | 80nm |
Conclusion
In order to study the aerosol behaviour in the Reactor Containment Building (RCB), the bulk motion of aerosol under the gravity has a significant contribution. In this context, particle shape and its influence in deposition velocity of nano sized strontium peroxide particles has been studied.
References
1.Subramanian.V, Baskaran.R, Krishnan. H, (2009) ‘Thermal Plasma Synthesis of Iron Oxide Aerosols and their Characteristics’ Aerosol and Air Quality Research, Vol. 9, No.2, pp.172-186.
2.Fendel.W, Th.Kauffeldt, Schmidt-Ott. A, (1995) ‘Measuring Properties of Nanoparticles by Aerosol Methods’, Nanostructured Materials, Vol.6, pp.655-658.
bThe strontium peroxide has been chosen for the study, since; it is a fission product material. The Strontium
(Sr) is stable as 88Sr38. It is formed as 90Sr during fission. It decays to 90Y (yttrium) as 38Sr90 38Y90 + -1e0 (546 keV). The fission yield of strontium 90Sr is high and it is a beta emitter having high effective half life time.
The inhalation of strontium peroxide aerosols may cause inflammation and irritation to the lungs and mucus membrane and behaves as a bone seeker.
282
IASTA-2010
C–O–3
Variations of Ion Pair Production Rate and Inhalation Dose due to Radon and its Progeny at Different Locations in Mysore City India
M S Chandrashekara*, B M Rajesh, T S Shashikumar and L Paramesh
Department of Studies in Physics, University of Mysore, Manasagangothri,
Mysore-570 006, India
Email: msc@physics.uni-mysore.ac.in
Introduction
Most of the radionuclides naturally occurring in air are radon (222Rn) and its daughters released from 238U series. The behavior of radon and its daughter products in the environment is a complex and dynamic process. When the atoms of progeny are first produced they will be positive ions, they are very reactive and will mostly attach themselves to aerosol particles, water vapor, oxygen, trace gases and other solid surfaces. Exposure of human beings to higher concentrations of radon and its short-lived progeny for a long period leads pathological effects. Inhalation of short-lived radon progeny seems to be the most important component of the radiation exposure of the population from natural sources. When inhaled both attached and unattached radon progeny will be deposited in the respiratory tract. In the decay of the progeny like polonium, alpha particles are emitted, which irradiate the cells of the lung tissues.. Therefore the radioactive progeny, rather than radon itself, presents health hazard 1-3.
To obtain an accurate estimate of radon related lung cancer risk and to plan proper control measures the population dose must be determined. Radon and its progeny concentration in the lower surface at 1m-height with meteorological parameters were measured at three different locations in Mysore city, India.
Methodology
Low Level Radon Detection System
Estimation of radon concentration in the atmosphere was made using the Low Level Radon Detection System (LLRDS) following the well–established procedure4,5. Air sample is collected in an evacuated chamber and exposed a circular metallic disk of 5 cm diameter to the air containing radon inside the collection chamber. The disc is maintained at a negative potential of 800V with respect to the body of the chamber, which is grounded. Radon decay products, as they are produced, are known to be positively charged. The decay products formed inside the chamber get attracted to the disc which is negatively charged. The decay products are collected for 75 minutes. The disc is then taken out and counted for alpha activity typically for 5000 sec. The minimum detectable accuracy for
283
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
radon in LLRDS is as low as 1.7–8.8 Bq m–3, depending on the relative humidity conditions. The concentration of radon Rn (Bq m–3) is calculated with the expression:
Where C is the total number of counts observed during the counting period, E is the efficiency of alpha counting system, F is the efficiency of collection of RaA–atoms on the metallic disc, V is the volume of LLRDS chamber, Z is the correction factor for build up and decay of radon daughter atoms on the metallic disc during the exposure and counting period4,5.
Air flow meter
Radon progeny concentrations are measured using an air flow meter of 15 cm long and 1 cm diameter. Air is drawn through a glass fiber filter paper by means of a suction pump at a known flow rate. The radon progeny in air sample are retained on the filter paper. Total activity on the filter paper is measured at three different counting intervals of 2 – 5, 6 – 20 and 21 – 30 minutes. Activities of RaA (218Po), RaB (214Pb) and RaC' (214Po) are calculated using the equations5,6.
Where C1, C2 and C3 are the gross counts during the three counting intervals, E is the efficiency of alpha counting system and V is the sampling rate in liters per minute.
Ion pair production rate and inhalation dose
The total energy released 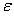 (eV cm–3 s–1) due to both radon and its progeny is computed from radon and its individual progeny concentrations and is used to calculate ion-pair production rate Q (No. cm– 3 s–1) (Ref. 7):
(eV cm–3 s–1) due to both radon and its progeny is computed from radon and its individual progeny concentrations and is used to calculate ion-pair production rate Q (No. cm– 3 s–1) (Ref. 7):
where Rn, RaA, RaB and RaC' are the concentrations (Bq m–3) of 222Rn, 218Po, 214Pb and 214Po respectively.
The inhalation dose due to radon and its progeny is estimated by using the formula5,8. Dose (m Sv y-1) = [0.17 + 9 × 0.6] C-R × 1760 ×10-6
Where, CR is the concentration of radon (Bq m-3).
284
IASTA-2010
The observations were made at (i) Maharani’s Science College Campus, Mysore city area (MSCM), which is in the vicinity of more anthropogenic activities and vehicular traffic (ii) Manasagangothri, University Campus, Mysore (MGM), covered with green grass and also less polluted area, and (iii) St. Joseph’s School Campus (SJS), an open field and very close to a main road where vehicles move frequently.
Results and discussions
Radon and its progeny concentrations
The diurnal variations of radon and its progeny concentrations were studied during April 2006-April 2007 at three locations in Mysore city (12oN, 76oE) and are shown in Tables 1 to 3. The result show that radon concentrations gradually increases and reach maxima in the early morning, 04–06 hrs and decrease after sunrise, attaining minima in the afternoon, 10 to 14 hrs at all locations.
Table 1. Diurnal variations of radon, its progeny activity concentration, and meteorological parameters measured at MSCM
| Time in | Concentration (Bqm-3) | IP | Dosem | Temp.oC | RH% | |||
| hours | cm3 s-1 | Sv. y-1 | ||||||
| Rn | RaA | RaB | RaC/ | |||||
| 0 | 4.07 | 13.25 | 1.76 | 0.19 | 3.26 | 0.04 | 20.0 | 83 |
| 2 | 20.47 | 11.90 | 0.84 | 0.31 | 5.83 | 0.20 | 20.5 | 82 |
| 4 | 28.68 | 9.02 | 0.96 | 0.54 | 6.76 | 0.28 | 27.0 | 64 |
| 6 | 22.78 | 13.56 | 0.84 | 0.19 | 6.53 | 0.22 | 30.0 | 49 |
| 8 | 10.25 | 6.72 | 1.22 | 1.22 | 3.34 | 0.10 | 21.0 | 82 |
| 10 | 9.10 | 9.02 | 1.34 | 0.80 | 3.49 | 0.09 | 21.5 | 82 |
| 12 | 10.37 | 4.30 | 0.35 | 0.54 | 2.72 | 0.10 | 24.0 | 69 |
| 14 | 6.49 | 6.72 | 1.00 | 0.70 | 2.57 | 0.06 | 25.0 | 69 |
| 16 | 9.25 | 4.49 | 0.84 | 0.96 | 2.69 | 0.09 | 28.0 | 59 |
| 18 | 6.14 | 3.61 | 0.73 | 1.38 | 2.08 | 0.06 | 28.5 | 53 |
| 20 | 7.30 | 3.77 | 0.58 | 1.12 | 2.23 | 0.07 | 26.0 | 63 |
| 22 | 5.88 | 14.90 | 1.76 | 0.07 | 3.88 | 0.06 | 23.5 | 76 |
| Avg. | 11.75 | 8.45 | 1.03 | 0.65 | 3.80 | 0.12 | 24.6 | 69 |
| Min | 4.07 | 3.61 | 0.35 | 0.07 | 2.08 | 0.04 | 20.0 | 49 |
| Max | 28.68 | 14.90 | 1.76 | 1.38 | 6.76 | 0.28 | 30.0 | 83 |
It is also interesting that the concentrations of radon follow the trend of the relative humidity, in general. This is due to the fact that as temperature increases, the humidity decreases resulting in the decrease of moisture content in the atmosphere9-10. This causes increased vertical mixing and rising of aerosols to the higher altitudes resulting in lower concentration of radon at the ground level. When the temperature decreases and humidity increases, the vertical mixing and raising of aerosols to the higher altitude reduces. As a consequence, the aerosol to which radon is attached, will be present at higher concentrations during night and in the early morning hours at ground level. This results in the increase of radon concentrations near the surface of the Earth.
285
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
The radon and its progeny concentrations were measured at MSCM, MGM, SJS and KSPCB locations are shown in Table 1-4. The radon concentration varies from 4.07 to 28.68 Bq.m-3 with an average of 11.75 Bq.m-3 in MSCM, it varies from 5.34 to 17.91 Bq.m- 3 with an average of 9.93 Bq.m-3 in MGM and 3.87 to 26.87 Bq.m-3 with an average of 10.18 Bq.m-3 in SJS and from 6.42 to 19.20 Bq.m-3 with an average of 10.43 Bq.m-3 in KSPCB. The higher radon concentration is found in MSCM location due to the higher radium content.
Table 2. Diurnal variations of radon, its progeny activity concentration and meteorological parameters measured at MGM
| Time in | Concentration (Bqm-3) | IP | Dosem | Temp.oC | RH% | ||||
| hours | cm3 s-1 | Sv. y-1 | |||||||
| Rn | RaA | RaB | RaC/ | ||||||
| 0 | 10.45 | 3.17 | 0.43 | 0.45 | 2.51 | 0.10 | 21.5 | 70 | |
| 2 | 11.57 | 2.92 | 0.31 | 0.48 | 2.97 | 0.11 | 21.0 | 75 | |
| 4 | 17.91 | 5.89 | 0.51 | 0.37 | 4.48 | 0.18 | 19.5 | 74 | |
| 6 | 17.28 | 5.94 | 0.67 | 0.32 | 4.36 | 0.17 | 23.0 | 82 | |
| 8 | 8.06 | 3.8 | 0.36 | 0.35 | 2.2 | 0.08 | 23.5 | 68 | |
| 10 | 5.61 | 3.02 | 0.32 | 0.45 | 1.46 | 0.05 | 28.5 | 59 | |
| 12 | 5.34 | 4.60 | 0.34 | 0.40 | 1.31 | 0.05 | 30.0 | 51 | |
| 14 | 5.72 | 2.92 | 0.38 | 0.14 | 1.58 | 0.06 | 31.5 | 43 | |
| 16 | 7.70 | 3.08 | 0.34 | 0.42 | 1.82 | 0.08 | 33.0 | 48 | |
| 18 | 9.95 | 2.22 | 0.63 | 0.65 | 2.29 | 0.10 | 29.5 | 49 | |
| 20 | 10.50 | 4.78 | 0.43 | 0.13 | 2.74 | 0.10 | 24.5 | 65 | |
| 22 | 9.00 | 6.44 | 0.63 | 0.16 | 2.61 | 0.09 | 22.5 | 75 | |
| Avg. | 9.93 | 4.06 | 0.44 | 0.36 | 2.53 | 0.10 | 26.0 | 63 | |
| Min | 5.34 | 2.22 | 0.31 | 0.13 | 1.31 | 0.05 | 19.5 | 43 | |
| Max | 17.91 | 6.44 | 0.67 | 0.65 | 4.48 | 0.18 | 33.0 | 82 | |
Where,
Dose: Outdoor Inhalation dose (mSv.y-1)
IP: Ion pair production rate due to radon and its progeny (no.cm3 s-1)
RH: Relative Humidity (%)
Ion pair production
The Ion pair production due to radon and its progeny concentrations at MSCM, MGM, SJS and KSPCB locations are shown in Tables 1-4. The Ion pair production due to radon and its progeny concentrations varies from 2.08 to 6.76, 1.31 to 4.48, 1.79 to 5.22 and 1.98 to 5.74 ion pair’s cm-3s-1 with an averages of 3.80, 2.53, 3.20 and 3.40 ion pairs cm-3s-1 at MSCM, MGM, SJS and KSPCB locations respectively.
Inhalation doses due to radon and its progeny
The inhalation dose varies from 0.04 to 0.28 m Sv y-1 with an average of 0.12 at MSCM, 0.05 to 0.18 m Sv y-1 with an average of 0.10 at MGM, 0.04 to 0.26 m Sv y-1 with an average of 0.10 m Sv y-1 at SJS location and 0.06 to 0.19 m Sv y-1 with an average of 0.10
286
IASTA-2010
Table 3. Diurnal variations of radon, its progeny activity concentration and meteorological parameters measured at SJS
| Time in | Concentration (Bqm-3) | IP | Dosem | Temp.oC | RH% | |||
| hours | cm3 s-1 | Sv. y-1 | ||||||
| Rn | RaA | RaB | RaC/ | |||||
| 0 | 13.26 | 7.90 | 1.00 | 0.37 | 3.87 | 0.13 | 22.0 | 75 |
| 2 | 14.52 | 4.03 | 1.23 | 0.81 | 3.48 | 0.14 | 22.0 | 75 |
| 4 | 12.80 | 6.59 | 1.49 | 0.67 | 3.64 | 0.13 | 21.5 | 74 |
| 6 | 26.87 | 2.26 | 0.97 | 0.69 | 5.22 | 0.26 | 21.0 | 74 |
| 8 | 8.71 | 10.99 | 1.27 | 0.22 | 3.64 | 0.09 | 25.5 | 84 |
| 10 | 3.87 | 8.00 | 1.56 | 0.71 | 2.12 | 0.04 | 38.0 | 27 |
| 12 | 5.43 | 3.76 | 1.41 | 0.48 | 1.79 | 0.05 | 32.0 | 51 |
| 14 | 4.71 | 6.22 | 1.01 | 0.40 | 2.10 | 0.05 | 34.0 | 43 |
| 16 | 6.59 | 5.02 | 1.65 | 0.29 | 2.18 | 0.06 | 35.0 | 31 |
| 18 | 4.88 | 24.53 | 2.78 | 0.00 | 4.67 | 0.05 | 30.5 | 49 |
| 20 | 6.23 | 12.18 | 1.17 | 0.45 | 2.43 | 0.06 | 25.0 | 77 |
| 22 | 14.30 | 4.00 | 0.78 | 0.39 | 3.32 | 0.14 | 22.5 | 75 |
| Avg. | 10.18 | 7.96 | 1.36 | 0.46 | 3.20 | 0.10 | 27.4 | 61 |
| Min | 3.87 | 2.26 | 0.78 | 0.00 | 1.79 | 0.04 | 21.0 | 27 |
| Max | 26.87 | 24.53 | 2.78 | 0.81 | 5.22 | 0.26 | 38.0 | 77 |
Table 4. Diurnal variations of radon, its progeny activity concentration and meteorological parameters measured at KSPCB-Building
| Time in | Concentration (Bqm-3) | IP | Dosem | Temp.oC | RH% | |||
| hours | cm3 s-1 | Sv. y-1 | ||||||
| Rn | RaA | RaB | RaC/ | |||||
| 0 | 9.89 | 7.54 | 0.86 | 0.46 | 3.67 | 0.10 | 21.0 | 91 |
| 2 | 11.02 | 14.59 | 1.75 | 0.46 | 4.80 | 0.11 | 21.5 | 91 |
| 4 | 15.34 | 15.72 | 1.63 | 0.31 | 5.69 | 0.15 | 24.0 | 91 |
| 6 | 19.20 | 11.64 | 1.30 | 0.96 | 5.74 | 0.19 | 25.5 | 83 |
| 8 | 9.68 | 7.87 | 1.29 | 1.02 | 3.42 | 0.09 | 23.0 | 76 |
| 10 | 8.11 | 5.55 | 0.70 | 0.56 | 2.59 | 0.08 | 29.5 | 76 |
| 12 | 7.02 | 4.59 | 0.56 | 0.51 | 2.21 | 0.07 | 28.0 | 77 |
| 14 | 6.56 | 4.67 | 0.77 | 0.69 | 2.19 | 0.06 | 29.5 | 91 |
| 16 | 6.42 | 3.38 | 0.66 | 0.98 | 1.98 | 0.06 | 31.5 | 83 |
| 18 | 9.27 | 5.50 | 0.50 | 0.50 | 2.76 | 0.09 | 29.0 | 91 |
| 20 | 11.91 | 5.23 | 0.42 | 0.17 | 3.08 | 0.12 | 25.5 | 82 |
| 22 | 10.71 | 3.81 | 0.46 | 0.49 | 2.67 | 0.10 | 22.5 | 82 |
| Avg. | 10.43 | 7.51 | 0.91 | 0.59 | 3.40 | 0.10 | 26.0 | 84 |
| Min | 6.42 | 3.38 | 0.42 | 0.17 | 1.98 | 0.06 | 21.0 | 76 |
| Max | 19.20 | 15.72 | 1.75 | 1.02 | 5.74 | 0.19 | 31.5 | 91 |
287
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
m Sv y-1 at KSPCB location. Calculation indicates that the dose due to inhalation of radon daughters is not very significant in Mysore. The mean outdoor inhalation dose to the population of Mysore is found to be 0.11mSv y-1 and it is comparable with the values obtained at other parts of the world8.
Conclusion
Diurnal variation of radon and its progeny concentration shows similar trend at all the locations showing a maximum during early morning hours from 04 – 06 hrs and minimum during from 10 – 14 hrs. The higher radon concentration is found to be in MSCM location due to the higher radium content. The mean outdoor inhalation dose to the population of Mysore is found to be 0.11mSv y-1 and it is comparable with the values obtained at other parts of the world.
References
1)Cliff, K.D, (1978). Physics in medicine and Biology, 23, 696-711.
2)Grasty (1994) Health Physics, 66(2), 185-193.
3)Sannappa, J., Paramesh.L. andVenkataramaiah.P., (1999). Indian Journal of pure Physics, 73 B(4), 629- 639.
4)Srivastava, G.K., Raghavayya, M. and Khan, A.H., (1984). Health Physics 46, 225-228
5)Chandrashekara M.S., Sannappa J. and Paramesh L., (2006). Atmos. Env., 40, 84-95.
6)Raghavayya, M., (1998), Radiation Protection Environment. 21, 127-132.
| 7) | Hoppel W A, Anderson R V and Willet J C, In The earth’s electrical environment, | National Academy |
| Press, Washington DC USA, (1986) 149–165. |
8)UNSCEAR (2000), Sources and Effects of Ionizing Radiation, Report to the UN General Assembly with Scientific Annexes.
9)Schubert M and Schulz H, (2002) Health Phys, 83; 1, 91–96.
10)Dhanorkar S, Deshpande C G and Kamra A K, (1989), Atmos Environ, 23, 839–841.
288
IASTA-2010
C–O–4
Can Ionizers Reduce Effective Inhalation Dose due to Thoron Progeny?
Manish Joshi, Arshad Khan, Pallavi Kothalkar and B K Sapra
Radiological Physics and Advisory Division
Bhabha Atomic Research Centre, Mumbai – 400 085, India
ABSTRACT : Ionizers are commonly used to control pollution in home as well as in personal vehicles. These have also been proven effective in reducing the activity concentration of radioactive aerosols in workplace environments. However, the physics of removal while using ionizers has been discussed in limited studies. The role of fine fraction and coarse fraction and their effect on the removal mechanism has been scarcely discussed. The increase of unattached fraction in the inhalable environment poses a doubt on the capability of the ionizers in reducing the effective dose due to the radon/thoron progeny because of the relative increase of dose conversion factor in that range. In this study, effectiveness of the ionizers in reducing the inhalation dose has been presented.
Introduction
The technique of ionizer based electrical particle removal has been tested on radioactive aerosols in some limited studies .The concentration reduction factor was shown to be varying from 3 to more than 7 in these studies. The removal mechanism has been discussed theoretically as a result of space charge induced electric fields by Mayya et al. (2004).This was extended by Joshi et al. (2009) by including a parametric study of thoron progeny concentration depletion. The physical arguments suggested to explain the reduction in concentrations are 1) direct plate-out of freshly formed, charged fine fraction of progeny through drift in the electric field and 2) removal of the coarse fraction, thereby increasing the highly mobile fine fraction and its consequent plate-out. However, the increase of unattached fraction (2% to 6.5% in our laboratory experiments) in the inhalable environment and increase of deposition velocity on the sensors placed on the wall (0.06 m/hr to 0.3 m/ hr in the same experiment) strongly favors the coarse fraction movement resulting in activity reduction (Joshi et al. 2009). This increase of unattached fraction and its effect on effective dose has been a topic of arbitration. Hence, the question, whether the ionizer reduces the effective dose or not, needs to be addressed quantitatively. This study relates all the ionizer induced parametric changes i.e. gross activity change, aerosol concentration change, and unattached fraction change to the most important quantity for radioactive aerosol studies i.e. effective dose change. We measured the activity concentration reduction factor of ionizers and unattached fraction increase and relate these parameters with the dose conversion factors. The effective dose calculation has been done as a part of the study and the MCRF (minimum concentration reduction factor) has been framed as a function of increase of unattached fraction.
The model for dose calculations used in this study was discussed by Ishikawa et al. (2007) which is based on the ICRP 66 respiratory tract model. This model expresses dose
289
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
conversion factor (nSv/hr/Bq/m3) as a function of particle size in two different ranges from 1 to 10 nm (AMTD) and 10 nm to 10000 nm (AMAD). It was proved in the formulation that, the lung effective dose is dominated by the contribution to the effective dose and the effective dose per EETC (equilibrium equivalent thoron concentration) was about four times larger than the effective dose per EERC (equilibrium equivalent radon concentration). The authors have pointed the need for EETC measurements to estimate the accurate dose. We conducted experiments and related our data with the model presented by the authors. The effective dose calculation was carried out using a wide range of parameters and the results were compared with the experiments performed in our lab.
Experiments
A systematic study has been carried out in a room environment (20 m3 volume). The particle concentration was measured with the help of Grimm 5.403 Scanning Mobility Particle Sizer. The activity concentration was measured by using gross activity sampler while unattached fraction was measured by using wire-screen based unattached fraction sampler. The thorium nitrate powder source was placed in the room with a fan inside while all other instruments were operated from outside. Ionizers were kept inside the room and their effect has been analyzed using the change in the gross activity concentration, unattached fraction and aerosol concentration. Few other similar experiments have also been carried out in the chamber, room and the field conditions to investigate the phenomenon. Unattached fraction increased and the total activity concentration was found to reduce in all experiments. The effective dose calculations were done using the dose conversion factors for thoron decay products.
| Results and discussion | |
| The results presented in this paper are for a few | |
| of the experiments done as a part of this study. As | |
| shown in fig 1, concentration reduction factor was | |
| around 5 with a mean life of 3.5 min. | |
| This reduction was the result of ionizers operating | |
| in the room of 20 m3 volume wherein the unattached | |
| fraction increased from 2% to 6.5 %. If A1 is the | |
| 3 | |
| activity concentration (Bq/m ) without ionizer, A2 is | |
| the activity concentration (steady state) with ionizer, | |
| p1 is the unattached fraction without ionizer and p2 | Figure 1. Activity concentration reduction |
| is that with ionizer than the dose reduction factor | |
| (ratio of pre and post ionizer dose) can be defined by | |
| or |
With K= A1/A2 as the activity reduction ratio and c=p2/p1 as the unattached fraction increase ratio. D1, D2, D1’ & D2’ are the dose conversion factors for the median of the unattached and attached fraction pre and post ionizer respectively.
For an advantageous effect of the ionizer, DRF should be greater than 1 and it can be analyzed as a function of K by varying unattached fraction (p1) and unattached fraction increase ratio (c).
290
IASTA-2010
The Dose conversion factor (nSv/hr/Bq/m3) variation has been incorporated in this formulation using the effective dose per unit exposure represented in the work by Ishikawa et al (2007). The activity median diameter for the coarse fraction was calculated in our experiment, it increased from 93.9 nm (GSD=2.6) to 148.9 nm (GSD=2.1). The dose conversion factor for these values were approximately150 nSv/hr/Bq/m3 & 100 nSv/hr/ Bq/m3 respectively. As the unattached fraction was measured using wire screen sampler, we can only approximate the median of the fine spectrum. As, the questionable issue is the increase of unattached fraction and consequent effect on the effective dose, we selected the most conservative median values for this range. We selected the minimum value of dose before ionizer application and maximum once it is in use. These values are approximately 210 nSv/hr/Bq/m3 for pre-ionizer and 770 nSv/hr/Bq/m3 for post-ionizer spectrum. Since the study aims at calculating effective dose change, these values will be most appropriate to use here.
| Figure 2a. p=0.02 | Figure 2b. p=0.05 |
Figure 2c. p=0.01 Figure 2d. p=0.02
Figure 2. Variation of dose reduction factor with increase in unattached fraction
Fig 2a to 2d shows the dose reduction factor calculation for the unattached fraction (p) of 0.02, 0.05, 0.1 & 0.2 respectively. DRF’s has been evaluated with increase in unattached fraction at fixed concentration reduction factor. It can be inferred that, except at most
291
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
unrealistic conditions (like in 2d when p=0.2 and c=4), DRF is always greater than 1 at feasible values of CRF.
Similar information can be deduced from three-dimensional plot (Fig 3) when p is varying from 0.01 to 0.2 and c from 1 t0 10 at concentration reduction factor k=3 (which is below the average experimental CRF).
Figure 3. 3D plot showing DRF as a function of p & c
For the experiment carried out in the lab, Dose reduction factor 5.27 was achieved (when concentration reduced by 5 times and unattached fraction increases from 2 to 6.5 %). As the above calculations were produced at conservative extreme, the effective dose is found to be reducing for most of the experimental situations.
Conclusion
The study proves that, the increase of unattached fraction and hence suspected effective dose increase can easily be offset by enhancing activity reduction ratio. As seen in our lab study, for the actual cases, DRF will be significant enough to prove the utility of ionizers as an effective tool. Even at the extreme cases, if activity reduction ratio (K) is more than a critical value (minimum activity reduction ratio), DRF will be more than one. This study can be used as a verification to place ionizers as a tool to reduce effective dose.
References
1.Y. S. Mayya, B. K. Sapra, Arshad Khan, Faby Sunny (2004), Aerosol reduction by unipolar ionization in indoor environments, Journal of Aerosol Science 35 923-941
2.M. Joshi, P. Kothalkar, A. Khan, R. Mishra, B.K. Sapra and Y.S. Mayya (2009) Parametric study of the ionizer induced Thoron progeny concentration depletion, European Aerosol Conference 2009, Karlsruhe, Abstract T122A0
3. Ishikawa T., Tokonami S. & Nemeth C. 2007 Calculation of dose conversion factors for thoron decay products, J. Radiol. Prot. 27 (2007) 447-456
292
IASTA-2010
C–O–5
Inhalation Dose due to Indoor Radon
S Sundareshan1, L A Sathish2*, H C Ramanna2 and K Nagaraja3
1Department of Physics, Vijaya Degree College, Bangalore – 560 004
2Department of Physics, Government Science College, Bangalore – 560 001
3Department of Physics, Bangalore University,Bangalore – 560 056
*Email: Sathish – lasgayit@yahoo.com
ABSTRACT : The 222Rn and 220Rn enter the atmosphere mainly by crossing the air interface of either soil-air or building materials. The existence of relatively high indoor concentration maintains a large 222Rn, 220Rn concentration gradient between the materials and open air. Solid state nuclear track detector (SSNTD) based dosimeters were used for the measurement of indoor 222Rn, 220Rn. The present paper deals with the experimental measurement of 222Rn, 220Rn concentrations and the associated inhalation dose rates due to them in the dwellings of different characteristics. Higher dose rates were observed in lower volume houses and the houses with granite flooring.
Introduction
In recent years the concern has developed about possible associations of indoor radon exposure with cancers. Outstanding issue now is the health effects of low levels of chronic radiation exposures. So far on definite trends are observed in the available studies and there exist a large variability in the effects obtained in different facilities and cohorts. Results of continuing studies would eventually lead to a definite picture of the corresponding dose response relationships and the risk estimates. In view of this, it is essential to have a clear image of these and an attempt is made to estimate the effective dose rates due to the exposure of indoor 222Rn, 220Rn and their progeny levels to the population of Bangalore city, India. Also, the observations were made to study the seasonal variations, dependence on different walls, floorings, rooms and range of volume of the houses. This type of study strengthens the results for specific area and adds up as a data bank for radon measurements.
Methods and Measurements
Twin cup dosimeters developed in Bhabha Atomic Research Centre, Mumbai were used. The dosimeter has two cylindrical cups of equal volumes having radius 3.1 cm and height 4.1 cm. The cups are having provision to hold SSNTD films inside the cups and a third film outside the cup for progeny measurements. The dosimeter is designed to discriminate 222Rn and 220Rn in mixed field situations, where both the gases are present like in monazite rich deposit areas. Track detector used in the dosimeter is cellulose nitrate films, commercially called LR-115 films. Films of size 3×3 cm2 were affixed at the bottom of each cup as well as on the outer surface of the dosimeter. The exposure of the detector inside the cup is termed as cup mode and other one exposed openly is termed as bare mode. One of the cups has its entry covered with a glass fiber filter paper that permeates both 222Rn and 220Rn gases into the cup and is called filter cup. The other cup is covered with a
293
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
semi permeable membrane sandwiched between two glass fiber filter papers called membrane cup. These types of semi permeable membranes have diffusion coefficient for radon gas in the range of 10–8–10–7 cm2 s–1 that permeates more than 95% of the 222Rn gas while it suppress the entry of 220Rn gas to more than 99%. Thus, the SSNTD films inside the membrane cup register tracks that attributes to 222Rn gas alone, while the filter film records tracks due to both 222Rn and 220Rn gases. The third film exposed in the bare mode registers alpha tracks produced by both the gases and their alpha emitting progeny. After exposure, the dosimeters were retrieved and SSNTD films were removed from the dosimeter for etching. The films were then etched in 10% NaOH solution at 60 ºC for 90 min Eappen and Mayya (2004). The tracks recorded on LR-115 films were counted using a spark counter. Tracks are converted to gas concentrations using Equations (1) and (2).
(1)
(2)
where Tm is track density of film in membrane compartment (Tr cm-2), d is period of exposure in days (d), Sm refers to sensitivity factor of membrane compartment (Tr cm-2)/ (Bq d m-3), Tf is track density of film in filter compartment (Tr cm-2), Srf is the Sensitivity of 222Rn in filter compartment (Tr cm-2)/(Bq d m-3) and, CR and CT are the concentrations (Bq m-3) of 222Rn and 220Rn, respectively. Followed protocol of Eappen and Mayya (2004) for processing the exposed films; hence sensitivity factors Sm and Srf are taken from their work for computing the gas concentrations. The progeny concentrations in terms of Working Level (WL) can be written as:
(3)
(4)
where FR and FT are equilibrium factors for 222Rn and 220Rn respectively and can be equated with progeny fractions of respective gases as shown in Eqs. (5) and (6).
(5)
(6)
where FRA, FRB, FRC, FTB and FTC are activity fractions of 218Po, 214Pb, 214Bi, 212Pb and 212Bi, respectively. Mayya et al (1998) have obtained these activity fractions through ventilation parameters applying a root finding method using the deposition velocities for attached and unattached fractions of the progeny nuclides. In the present study, inhalation dose is computed using F values of UNSCEAR (2000). Indoor occupancy factor for the population is taken as 0.8 and the annual inhalation dose (mSv y–1) is calculated using Eq. (7).
(7)
294
IASTA-2010
Results and Discussion
About 150 dwellings in ten different locations of Bangalore city, India were selected on the basis of construction, age of the building, nature of walls, rooms and floorings to see the effective dose rates due to indoor 222Rn, 220Rn and their progeny levels in dwellings during different seasons of the year, and also for different walls, floorings, rooms and different volume of the houses.
Seasonal variations and dose rates : The mean values of 222Rn and 220Rn concentrations of all the studied locations during different seasons are shown in Fig. 1(a) and the annual effective dose rates in Fig. 1(b). The higher concentrations observed during winter season is may be due to the radioactive gases are trapped near to the surface because of temperature inversions. In summer, the higher rate of vertical mixing and dispersions lifts the aerosols to higher altitudes resulting in a decrease in the concentration near the ground level air (Sesana et al 2003). Magalhaes et al (2003) have observed a two order of magnitude of variability, with a maximum of 50 Bq m-3 in winter and a minimum of 0.5 Bq m-3 in summer months. In addition radon exhalation rate also decreases during monsoon as soil pores get filled by water and hence, resulting in lower concentration of 222Rn and 220Rn (Nagaraja et al 2003).
| Figure 1. | Season wise 222Rn, 220Rn and dose rates | Figure 2. Wall wise 222Rn, 220Rn and dose rates |
Wall wise variations and dose rates : The mean concentration of 222Rn and 220Rn levels in different walls are shown in Fig. 2(a) and the respective dose rates in Fig. 2(b). Higher concentrations and dose rates were observed in mud wall houses and lower in concrete walls. The concentrations were found to vary from wall to wall. The variation is may be due to the random distribution of radioactive rock species used ignorantly in the construction of houses.
Floor wise variations and dose rates : The higher concentrations of 222Rn and 220Rn were observed in granite flooring houses and lower in mosaic flooring and are shown in Fig. 3(a). Granite is rich of radium and it may be the reason for higher concentration of radon in granite flooring houses. The materials used for construction of buildings are sufficiently porous and allow radon to enter into the indoor atmosphere (Gaso 2005). Granite samples show higher radon exhalation rate than mosaic. There is a positive correlation between radium content of granite with radon exhalation and its concentration (Al-Jarallah 2001). The variations of dose rates in different floorings are shown in Fig.
295
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
3(b). The elevated levels were observed in the granite floorings and the lower in mosaic floorings.
| Figure 3. Floor wise 222Rn, 220Rn and dose rates | Figure 4. Room wise 222Rn, 220Rn and dose rates |
Room wise variations and dose rates : Variations of indoor 222Rn and 220Rn concentrations in different rooms of houses are shown in Fig. 4(a). The high concentrations were observed in bath room, bed room and lower in living rooms. One can clearly see in the figure that there is soaring concentration in bathroom compared to the other rooms of the houses. Bed rooms might be expected to be least ventilated, on the average based upon limited use patterns and bath rooms may receive some additional 222Rn due to 222Rn dissolved in water (Sathish et al. 2006). 222Rn is shown to be released in spray from faucets or shower fixture (Gessel 1980). Air in living rooms on the other hand is most readily diluted due to outdoor air blow. The variations of dose rate in different rooms of the houses are shown in Fig. 4(b). Higher dose rates were observed in bed room and bath rooms.
Volume wise variations and dose rates : The volumetric variations of 222Rn and 220Rn are shown in Fig. 5(a). Higher concentrations were observed in lower volume room than in the higher volume room. The concentrations in a dwelling of volume 30-310 m3 ranged from 4 to 93Bq m-3. It is interesting to note that as the volume of room varies in geometric
| progression; there is no linear dependence | ||||
| on the concentrations. This clearly indicates | ||||
| that though the observations have been | ||||
| made almost for similar type of | ||||
| constructions and lifetime of the houses, | ||||
| but as the volume of the room increases | ||||
| the concentrations reduces exponentially | ||||
| and it becomes almost constant above the | ||||
| house volume 150 m3. The regression | ||||
| coefficients for the exponential drops for | ||||
| 222Rn and 220Rn are 0.99 and 0.98 | ||||
| respectively. The variations of dose rates | ||||
| are shown in Fig. 5(b). The present work | ||||
| 222 | 220 | reveals that the dwellers of lower volume | ||
| Figure 5. Volume wise | Rn, | Rn and dose rate | ||
296
IASTA-2010
houses will expose themselves to the higher dose rates and is 4.4 times of the dose received in higher volume houses.
Conclusions
With the aim of estimations of dose rates in dwellings of different features. The higher values were observed in lower volume house, granite flooring house, bath room, mud wall houses and in winter season. Among these the lower volume and granite flooring house inhabitants are exposed to higher dose. Hence, it is recommended that the lower volume houses should have good ventilation to reduce the effective dose rate.
Acknowledgments
The research work is sponsored by the University Grants Commission in the form of research grants under the Research Funding Council for major research project, X Plan, UGC, New Delhi. The cooperation extended by all the residents is highly appreciated and the support extended by all the principals of government science college, Bangalore for allowing us to carry out the research work in the college is highly acknowledged.
References
Al-Jarallah, “222Rn exhalation from granites used in Saudi Arabia” J Env Radioact 53(2001) 91-98.
Eappen K P and Mayya Y S, “Calibration factors for LR-115 (type-II) based radon thoron discriminating dosimeter” Radiat Meas 38 (2004) 5-17.
Gaso M I, Segovia N, Pulinets S, Leyva A, Ponciano G, Pena P, “Indoor radon and annual effective doses at a high altitude region in central Mexico” J Appl Sci, 5(2005)1356-1362.
Gessel T F and Prichard H M, “The concentration of 222Rn in tap water to Indoor 222Rn concentrations” In: Natural Radiation Environment-III, Eds. Gessel TF, Prichard HM, Springfield, (1980) 1347-1363.
Magalhaes M H, Amaral E C S, Sachett I and Rochedo, “Radon-222 in Brazil: an outline of indoor and outdoor measurements” Journal of Environmental Radioactivity ERR, 67 (2003) 131-143.
Mayya Y S, Eappen K P and Nambi K S V, “Methodology for mixed field inhalation dosimetry in monazite areas using a twin cup dosimeter with three track detectors” Radiat Prot Dosim 77 (1998) 177-181.
Nagaraja K, Prasad B S N, Madhava M S, “Chandrashekara MS, Paramesh L, Sannappa J, Pawar SD, Murugavelu P and Kamra AK” Radiat Meas 36(2003) 413-417.
Sathish L A, Sannappa J, Paramesh L, Chandrashekara M S and Venkataramaiah P, “Studies on the concentrations of radon/thoron and their progeny in houses of different types of floorings in Mysore city” Env Geochem 9(2006) 105-108.
Sesana L, Caprioli E and Marcazzan G M, “Long period study of outdoor radon concentration in Milan and correlation between its temporal variations and dispersion properties of atmosphere” Journal of Environmental Radioactivity, 65(2003) 147-160.
UNSCEAR. Report to the General Assembly with Scientific Annexes, United Nations. Annexure B, pp. 84 -156 (2000).
297
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
C–O–6
Studies on Bi-polar Charging of Strontium Peroxide Aerosols in the Presence of Gamma Field
V. Subramanian, R. Baskaran, J. Misra and P. Chellapandi
Radiological Safety Division, Safety Group
Indira Gandhi Centre for Atomic Research, Kalpakkam – 603 102. India
Introduction
In the case of core disruptive accident condition of fast reactor, fuel, fission products and sodium (coolant) aerosols are released into reactor containment building (RCB) and they remain suspended in the containment volume. In the presence of gamma radiation field, the aerosols acquire bi-polar charges. When these aerosols coagulate with acquired bi-polar ions, an additional electrical force act between them, resulting enhanced coagulation [1]. In order to understand the enhanced coagulation process, it is important to know the charge-size distribution of the aerosols. In this study, experiments are carried out in Aerosol Test Facility (ATF) for measuring the charge-size distribution of SrO2 aerosols in the presence of gamma radiation field and the mean number of charges acquired is determined. Studies on mean charge for the distribution of aerosols for various number concentrations and dose rates are presented.
Experimental
Aerosol Test Facility (ATF)
The schematic diagram of ATF is shown in Fig.1. The ATF consists of (i) Aerosol Chamber, (ii) Aerosol generators viz. Sodium combustion cell for the generation of sodium aerosols and Plasma Torch for the generation of fission product aerosols, (iii) Aerosol Diagnostic equipments and, (iv) Auxiliary systems like Vacuum system, Chilled water system, Pneumatic control system, Data Acquisition system and Humidity controller. For the real time measurement of aerosol characteristics, various instruments like Optical Counters, QCM, Sequential Mobility Particle Sizer are connected to the chamber through various connecting ports. In order to measure the charge-size distribution, Electrical Low Pressure Impactor (ELPI) [2] (M/s Dekati Ltd., Finland) is integrated into the aerosol chamber in one of the arms. The aerosol ports are provided with nozzles for connecting various equipments, which satisfies the Davis criteria to ensure negligible sampling error for still air sampling condition for the particles below 20 m. The typical inner diameter of the sampling nozzle for ELPI is 13mm.
Results and Discussion
A known weight of SrO2 powders (M/s ACROS Organics, USA) were palletized. Typical weight of the pellets were 5±0.2 g. The pellets were rigidly fixed in to the wire
298
IASTA-2010
feeder tube and exposed to plasma flame. The pellet melts and the molten vapours after leaving the flame zone condense to produce aerosols, which are flown into the aerosol chamber. By using ELPI with corona charger off condition, the charges associated with aerosols were measured as charge- size distribution. Also with corona charger on condition n u m b e r - s i z e distribution were measured. The
aerosol chamber volume is exposed panoramically with gamma radiation source (Cs-137), the charge-size distributions were measured with and without the presence of gamma radiation field. By using charge-size distribution, number-size distribution, the flow rate of the instrument and by taking the elementary charge value, the average number of charges associated with each sized aerosols are determined. The experiments were repeated with different aerosols number concentrations and dose rates.
Figure 2 represents average number of charges acquired by the distribution of SrO2 aerosols, i.e. each bar represents net charges measured for that particular size divided by the number concentration of that size. It is observed that, there exists the trend of increase in the average charge acquired by SrO2 aerosols when they are exposed to gamma radiation field. Similar trend is seen for two other different runs. Figure 3 represents the mean
| number of chargesa <j> (abs<j>) | ||||||
| acquired by the distribution of | ||||||
| aerosols, | versus | number | ||||
| concentration | of aerosols | (N | ||||
| cm-3) for the two cases viz. | ||||||
| without gamma field and with | ||||||
| gamma | field | and | the | |||
| experiments are repeated with | ||||||
| two different dose rates and the | ||||||
| corresponding | results | are | also | |||
| included in Figure 3. It is | ||||||
| observed that mean number of | Figure 2. Average number of charges <j> Vs particle diameter (d) | |||||
| charges <j> (abs<j>) | acquired | |||||
| m | ||||||
299
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
for the distribution of aerosols decreases with increase of number concentration of aerosols ‘N’, for a particular ion- pair concentration ‘n’. It is also observed, with increase in the value of ion-pair concentration ‘n’ for a given value of aerosol number concentration ‘N’, the mean charge ‘<j>’ value increases.
Particle charge distribution is represented by modified Boltzmann distribution (MBD)b [3], which takes into account of not only steady state charging condition but also ion- asymmetry. Fig. 4 represents the charge distribution of SrO2 aerosols for a Count Median Diameter of 0.51μm (for a typical value of number concentration ‘N’). It shows that the maximum of the charge distribution is around -1 charge for the ion-asymmetry of 1 (for a typical value of ion-pair concentration ‘n’).
Summary and Conclusion
Figure 3. Mean Charge acquired by the distribution of aerosols Vs Number concentration for the two cases with and without gamma field and two gamma dose rates (0.04 Gy/h and 0.48Gy/h)
Figure 4. Charge distribution estimated by MBD Theory
In the case of non-radioactive aerosols exposed to gamma radiation, each sized aerosols acquire distribution of charges and the corresponding mean charge <j> acquired by aerosols increases with increase in ion-pair concentration (n).
a The mean charge on a collection of particles is defined as
| where | where p = specified cut-off level |
b The aerosol charge associated with these ion concentrations can be determined from the modified
Boltzmann distribution as
where, for a monodispersed aerosol of radius a, there is number concentration Nj of particles carrying j charges. n+ and n- are the bipolar ion concentrations with associated mobilities 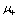 and
and 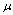 , e is
, e is
| modulus of the electronic charge, k is Boltzmann’s constant and | the permittivity of free space. The |
| corresponding mean charge <j> for the particles is | . |
300
IASTA-2010
References
1.V. Subramanian, R. Baskaran and R. Indira, “Experimental study on enhanced Brownian Coagulation of Sodium Compound Aerosol in the presence of Gamma Field”, Journal of Aerosol Science, Vol. 39, p.814 (2008).
2.Technical Specification and Operating Manual ELPI
3.C.F. Clement, R.A. Clement and R.G. Harrison, “Charge distributions and coagulation of radioactive aerosols”, Journal of Aerosol Science, Vol.26, p.1207 (1995).
301
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
C–P–1
Morphological Characteristics of Plasma Generated Aerosols
Arshad Khan1, Manish Joshi1, D Sen2, B K Sapra1 and Y S Mayya1
1Radiological Physics and Advisory Division, 2Solid State Physics Division
Bhabha Atomic Research Center, Mumbai- 400085, India
ABSTRACT : The particles generated through Plasma Torch are very often used as test aerosols for simulated reactor containment aerosol studies [1]. Characterisation of these particles are very important as particle diameter and density have great bearing on the particle behaviour in the containment. These parameters are also important in the aerosol codes used for probabilistic safety assessment studies of reactors, as they are assumed to be spherical particles with half of the original material density [2].
Plasma Torch is characterised by high enthalpy, high temperature and high reactivity, which brings changes in physical, chemical and metallurgical characteristics of the materials. The high concentrated enthalpy available in the plasma jet can vaporize virtually any refractory material and the high quench rate associated with the process suppresses grain growth, favours homogeneous nucleation resulting in ultra-fine particles [3]. In this process, commercially available metal or ceramic powder is fed into a dc non-transferred plasma torch. The particles are molten and leave the flame and fall through the atmospheric air inside a plasma reaction chamber. The particles are quenched, solidified and collected in a collection chamber. In-flight, if the particles are overheated, depending on the size, they may partially or fully vaporize. The smaller particles fly in all directions and get deposited on the walls of the reaction chamber. Vapors may also condense on the cooled walls.
In the present work, Zn metal powder of 70 m size was fed in to the plasma torch. The aerosols thus generated were collected using a wire mesh sampler. The structure of aerosols was then characterized by small-angle neutron scattering (Fig.1) and microscopy
| Figure 1. SANS data of Plasma Aerosol q represents | Figure 2. SEM of plasma aerosol |
| the wave vector transfer |
302
IASTA-2010
(Fig. 2). Scanning Electron Micrographs (SEM) showed highly porous or spongy structure with a primary particle size of around 70 nm, which is also confirmed by Small Angle Neutron Scattering (SANS). SANS also revealed that the aerosols have a fractal structure with fractal dimension of 2.75.
Reference:
[1]B. K. Sapra, Y. S. Mayya, Arshad Khan, Faby Sunny, Sunil Ganju, H. S. Kushwaha (2008) Aerosol Studies in a Nuclear Aerosol Test Facility Under Different Turbulence Conditions, Nuclear Technology, vol 163, no 2, 228-244
[2]NAUA Mod 5 Manual
[3]Cabanillas E.D. (2008) Oxidized iron nanoparticles obtained by high-power plasma cutting, Material letters, vol. 62, 4443-4445.
303
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
C–P–2
Indoor 220Rn and its Progeny Levels in a Dwelling
L A Sathish1*, K Nagaraja2 and V Nagesh1
1Department of Physics, Government Science College, Bangalore – 560 001
2 Department of Physics, Bangalore University, Bangalore – 560 056
* Corresponding author: E-mail: lasgayit@yahoo.com
ABSTRACT : In the traditional dwellings, source of 220Rn is the bare soil floor, either soil in cave dwellings or unburned adobe bricks and uncovered stone, wall in above ground dwellings. Because of the short half life of 220Rn, the indoor concentration is not homogeneous but increases towards the walls, floorings and ceilings. In view of this an extensive study is made by using the solid state nuclear track detector based dosimeters which were installed in parabolic fashion to see the variations of 220Rn and its progeny levels as a function of distance in a room of volume 30 m3. Higher concentrations were observed at the flooring, wall and ceiling of the room and it decreases as the detector is moved away from them. 220Rn progeny concentrations did not show any variations with the distance from the wall.
Introduction
The 220Rn has a short half-life, 55.6 seconds, compared to 222Rn. This means the distance that the 220Rn gas atoms can migrate in the ground, inside building materials and buildings before it decays is much shorter than 222Rn gas and also it is easily stopped by wall paper and other surface sealants. Therefore the risk for high 220Rn levels in indoor can be expected to be low, at least much lower than the risk for high levels of 222Rn. However, in buildings with an ineffective barrier between soil and indoor air the entry of 220Rn could be significant, especially if the gravel or the soil itself immediately under the building has a high concentration of 232Th. Soil as a significant source of indoor 220Rn has been demonstrated by Li et al (1992). Enhanced 220Rn levels were reported in residential traditional dwellings in India (Sreenath Reddy et al 2004) and in China (Shang et al 2005). The indoor 220Rn concentration is not only determined by the exhalation but also by the detector distance from the wall, ceiling and the flooring of the room. In the report of UNSCEAR (1993) the annual effective dose from 220Rn and its progeny was evaluated to be 75 μSv, only about 6% of that of 222Rn and its progeny. Measurements were performed in order to form a basis for assessing the risk for high indoor 220Rn levels of Bangalore city.
Methods and Measurements
Solid State Nuclear Track Detector based dosimeters were used for the measurement of thoron and its progeny concentrations. This is a good technique to study the long-term measurements taking into account the diurnal and seasonal variations of 222Rn and 220Rn concentrations (Fleischer 1975). The mode of sampling is passive and integrated. The detailed description of experimental methodology (Mayya et al 1998) and calibration procedure (Eappen and Mayya 2004) is available in the literature. The exposed detectors are alpha counted using spark counter. This technique is applicable to plastic detectors,
304
IASTA-2010
which provides a convenient, economical and fast method for track counting. This technique was developed by Cross and Tommasino (1970) and is discussed in detail by Samyogi et al (1978).
Results and discussions
The main objective of the study is to find the dependence of concentrations on distance and to assess the possible health hazards from indoor 220Rn levels in Bangalore city. Buildings were chosen regardless the natural 232Th concentrations. All the measurements were performed on the ground floor. The dosimeters were suspended in the room of volume 30 m3 in a lower and upper parabolic fashion shown in Figs. 1-2. Large numbers of dosimeters were suspended in particular fashion to reveal the actual information about the dependence of concentration as a function of distance. The results of the measurement of variations of 220Rn concentrations with floor distance are shown in Fig.3. The steep increase in concentration close to the floor or wall is observed and the concentration drops exponentially as the detector distance increases from the floor or wall and it may be due to its short half life. This suggests that it is necessary to keep the distance from the floor or wall when we measure indoor 220Rn concentration (Yamasaki 1995). It is evident from the Figure 3 that the 220Rn concentration is declining towards the room center and it may be because of the short half life of 220Rn and the time necessary for its transport (Yamasaki 1995). It is evident that the walls and floor of rooms were made of local soil material and bricks, which are the source of indoor 220Rn concentrations. Figs. 4 represent the vertical profiles of 220Rn concentrations, as the detector distance increases from the floor the concentration decrease exponentially. During the measurement period with twin cup dosimeters, the distribution of 220Rn progeny and 222Rn concentration were also measured at the different distance from wall and floorings. 220Rn progeny concentration was nearly independent of the distance from soil wall. The uniformity of concentrations in a dwelling is may be due to their long half life (Tso and Li 1987) and this was confirmed through model calculation (Tschiersch et al 2007). In contrast, the 222Rn concentration is homogeneous within the dwelling due to its longer half-life of 3.82 days. Close to the walls or floorings the 220Rn concentration is significantly higher. At increasing wall or floor distances, the 220Rn concentration may decrease but the 222Rn concentration remains steady. This type of observation was also made in several dwellings in the Gansu area (Shang et al 2005), so that it appears to represent a general feature of indoor 222Rn concentration. The turbulent transport from the wall into the room center decreases the
| Figure 1. Parabolic curve focus away from the floor | Figure 2. Parabolic curve focus away from the Ceiling |
305
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
relative contribution of the 220Rn close to the wall. This is important for the dose assessment of dwellers only at ventilation rates above the exhalation saturation the total activity declines (Zhuo et al 2000).
| Figure 3. Concentration profile of 220Rn | Figure 4. Vertical Distribution of 220Rn levels |
Conclusions
The concentrations were high near the wall and flooring of the room and it drops exponentially with the distance from wall and flooring. Indoor 220Rn progeny concentrations are uniform with the distance from the wall. Continuous and long-term studies such as diffusion of 220Rn from each wall of the building materials and factors that influences the 220Rn progeny levels in dwellings are necessary to assess the dose due to 220Rn and its progeny. More detailed studies on the evaluation of public exposure from the natural radiation; particularly the exposure from indoor 220Rn and its progeny should be planned and performed in the country.
Acknowledgements
The research work is sponsored by the X Plan of University Grants Commission, New Delhi in the form of research grants under the Research Funding Council for major research project. The cooperation extended by all the residents is highly appreciated and the support extended by all the principals of Government Science College, Bangalore for allowing us to carry out the research work in the test room is highly acknowledged.
References
Cross W G and Tommasino L, “Rapid reading technique for nuclear particle damage tracks in thin foils”
Radiation Effects, 5 (1970), 85–89.
Eappen K P and Mayya Y S, “Calibration factors for LR-115 (type-II) based radon thoron discriminating dosimeter” Radiation Measurements, 38 (2004), 5-17.
Fleischer R L, Price P B and Walker, “Nuclear tracks in solids” University of California Press, Berkeley, (1975), 421-435.
Li Y, Schery S D and Turk B, “Soil as a source of indoor 220Rn” Health Physics, 62 (5) (1992), 453–457.
Mayya Y S, Eappen K P and Nambi K S V, “Methodology for mixed field inhalation dosimetry in monazite areas using a twin cup dosimeter with three track detectors” Radiation Protection Dosimetry, 77 (1998), 177–181.
306
IASTA-2010
Samyogi G, Hunyadi I and Varga Z, “Spark counting of alpha radiograms recorded on strippable cellulose nitrate LR-115 film” Nuclear Track Detectors, 2 (1978), 191-197.
Shang B, Chen B, Gao Y, Cui H and Li Z, “Thoron levels in traditional Chinese residential dwellings”
Radiation Environment Biophysics, 44 (2005), 193-199.
Sreenath Reddy M, Yadagiri Reddy P, Rama Reddy K, Eappen K P, Ramachandran T V and Mayya Y S, “Thoron levels in the dwellings of Hyderabad city, Andhra Pradesh, India” Journal of Environmental Radioactivity, 73 (2004), 21-28.
Tschiersch J, Li W B and Meisenberg O, “Increased 220Rn indoor concentrations and implication to inhalation dosimetry” Radiation Protection Dosimetry, 127(1-4) (2007), 73-78.
Tso M W and Li C, “Indoor and outdoor 222Rn and 220Rn daughters in Hong Kong” Health Physics, 53(2) (1987), 175- 180.
United Nations Scientific Committee on the Effects of Atomic Radiations. Sources and Effects of Ionizing Radiation, (1993), 73. (New York: United Nations)
Yamasaki T, Guo Q and Iida T, “Distributions of thoron progeny concentrations in Dwellings” Radiation Protection Dosimetry, 59 (1995), 135–140.
Zhuo W, Iida T and Yang X, “Environmental Radon and Thoron Progeny Concentrations in Fujan Province of China” Radiation Protection Dosimetry, 87 (2000), 137- 140.
307
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
C–P–3
Effect of Aerosols on Back to Front Count Ratio of Unattached Thoron Progeny Deposited on Wire Screens
Rajni Modi, Manish Joshi, Pallavi Kothalkar, Arshad Khan and B K Sapra
Radiological Physics and Advisory Division
Bhabha Atomic Research Center, Mumbai- 400085, India
ABSTRACT : Diffusion batteries are used to capture and characterize the nanometer particles, which fall below the detection limit of most aerosol measuring instruments. Wire screens are used as the plates of diffusion batteries to facilitate the size segregation of this highly diffusive species. These wire screens have been often used in many studies to estimate parameters like unattached fraction of radon decay products, highly dispersive aerosol systems etc. There are a few theories available for wire screen in terms of its efficiency for capture of aerosol particles. In this paper, we present studies pertaining, front to back ratio of counts in wire mesh, used as the correction factor for alpha counting in these formulations in terms of variation in external parameters. Wire screens of 100 & 200 mesh have been used with flow rates from 1 to 4 lpm. The aerosol concentration was kept between 4000- 10000 per cc. The back to front count ratio was found to be increase 2.73 times, when fiber Reynolds number changes from 0.15 to 1.31. The results have been compared with the existing theories for the above correction factor and an under-prediction of the correction factor (count ratio) using available theories has been shown in presence of aerosols.
Introduction
The aerosol properties and their deposition processes depend mostly on the aerosol size. In radioactive environment, one important aspect is to study the deposition of the radioactive aerosols inside the lung and the consequently calculating the effective dose. The inhalation of radon and its progenies is a burden to radiological safety, particularly in the front end of the nuclear fuel cycle. The aerosol size spectrum of the working environment thus can be considered as the most relevant input to study the inhalation risk. In general, the radioactive progeny occurs in two specific size ranges; fine and coarse fraction. The relative ratio of these fractions varies with different environmental conditions as these conditions affect the fate of the progeny formed. The fine fraction relatively becomes more important due to its high probability of deposition in the lungs and hence needs to be estimated precisely. However, most of the aerosol instruments have their lower detection limit not less than 5- 10 nm. A systematic effort to capture and characterize the fine fraction has been made using diffusion batteries composed of an array of wire screens. Wire screens can be used to capture this sub-micrometer fraction using the property of large diffusivities of aerosols in this size range. Thomas & Hinchliffe (1972) measured collection efficiency of wire screens and also gave an equation for wire screen efficiency. Cheng- Yeh theory (1980) helped in selection and characterization of the wire screen. Solomen & Ren (1992) gave an expression for calculating front to total ratio as a function
308
IASTA-2010
of collection efficiency of screen. Mishra et al (2008) used the collection efficiency of wire screens in measurement and analysis of radon progeny deposition velocities.
Proper selection of the screen, its physical properties, air flow rates, design of the sampler and the correct counting protocols help in the correct utilization of the wire screen. Once characterized, the wire screen sampler can be used to measure the fine fraction which then can be used to estimate air activity and the inhalation dose risk. The aim of this work is to investigate few critical parameters of the wire screens and their dependence on some external parameters like diameter of wire screen, sampling flow rate etc. in presence of aerosols.
Front to back ratio (Canoba et al, 2000) for wire screen can be written as
Where 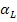 is the specific activity loss due to the interception of a particle. B and F denote the counts obtained experimentally on the back and the front end of wire screen exposed and counted simultaneously.
is the specific activity loss due to the interception of a particle. B and F denote the counts obtained experimentally on the back and the front end of wire screen exposed and counted simultaneously.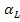 relates the number of particles intercepted by
relates the number of particles intercepted by
neighbor wires to the number of total particles emitted away from the wires surfaces. It can approximately be taken as 7% (Holub et al, 1987)
Solomen & Ren (1992) gave an empirical relation for front to total ratio as
| FT ratio = 0.67 | (e < 0.8) | ||
| = 3.56-7.17 e +4.45 e2 | (e >= 0.8) | (1) | |
e, the capture efficiency for the wire screen can be calculated using Cheng-Yeh theory or Alonso et al.’s (1997) work.
Experiments
Wire screens of 100 & 200 mesh size have been used in this work. Thorium nitrate powder source was placed in a chamber of 1 m3 volume with a stirrer and was allowed to be in equilibrium. The chamber was kept at moderate aerosol concentrations measured with the help of Grimm 5.403 CPC. The wire screens were mounted in samplers specially designed in our laboratory. This sampler has the facility to hold wire screen and filter paper accounting for rebounding of the captured active atoms. To calculate back to front count ratio, Y type flow divider, holding two wire screen samplers was exposed to the chamber environment. Front of one wire screen and the back of the second wire screen was counted in a ZnS (Ag) scintillation detector after same delay time.
Results
The physical characteristics of this wire screen (measured microscopically) are shown in table 1. Ratio of counts on backside of screen to the front side of screen as a function of Reynolds number for the fiber of the wire screen was calculated.
The single fiber efficiency was calculated using the formulation of Cheng-Yeh (1980) and Alonso et al. (2001).This efficiency was used to find the back to front count ratio
309
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
Table 1. Wire screen parameters
| Parameter | 200 mesh | 100 mesh | |
| Wire Diameter | 0.050 mm | 0.1 mm | |
| Gap (X axis) | 0.080 | mm | 0.144mm |
| Gap (Y axis) | 0.084 | mm | 0.162 mm |
| Mass | 0.096 gm | 0.229 gm | |
| Solid fraction | 0.429 | 0.478 | |
Figure 1. Comparison of Experiments with Theory
using FT ratio from eq(1). The aerosol concentration was kept on an average 7500 per cc during the measurements. Fig 1 shows the comparison of the experimental results with the theory.As apparent in the above figure, the Back to Front Ratio (B/F) for the wire screen increases with the fiber Reynolds number i.e. it increased from 0.247 to 0.923, when fiber Reynolds number changed from 0.1529 to 1.3104 in presence of moderate aerosol concentration. Fig.1 also shows B/F ratio calculated from the two theories mentioned above, wherein the aerosol concentration is nearly zero so that all the progeny are assumed to be unattached. The comparison clearly shows that experimentally calculated B/F ratios become very much different than the theoretically calculated values, in presence of aerosols.
Conclusions
These results indicate that, the wire screen parameters are required to be carefully examined before using them in the formulation and assessing any information about the fine fraction from it. The role of other aerosol properties apart from diffusion is required to be investigated in terms of their effect on the wire screen parameters. Even a small change (like sampling flow rate due to change in pump-wattage) can change the wire screen parameters to an appreciable extent. A refined theoretical interpretation may be required to explain the wire screen performance in presence of aerosols.
References
1.Thomas, J. W., & Hinchliffe, L. E. (1972), Filtration of 0.001 ìm particles by wire screens, Journal of Aerosol Science 3:387-393
2.Cheng, Y. S., & Yeh, H. C. (1980), Theory of a screen type diffusion battery, Journal of Aerosol Science, 11, 313-320
3.Solomen B. Stephen & Ren T. (1992), Counting efficiencies for alpha particles emitted from wire screens., Aerosol Science & Technology, 17, 69-83
4.Mishra, Rosaline, Mayya, Y.S. (2008), Study of a deposition based, Direct 220Rn Progeny Sensor (DTPS) technique for estimating Equilibrium Equivalent 220Rn Concentration (EETC) in indoor environment,
Radiation Measurements 43, 1408-1416.
310
IASTA-2010
5.Canoba C. A., Lopez O. F.(2000), Measurement of unattached fraction of 222Rn progeny using wire screen, Journal of Radioanalytical and Nuclear Chemistry, Vol. 245, No. 3 539-544
6.Holub, R. F., & Knutson, E. 0. (1987). In Radon and Its Decay Products: Occurrence, Propedies and Health Effects (P. K. Hopke, ed.). Symposium Series 331,American Chemical Society, Washington, DC, pp.340- 356
7.Alonso M., Kousaka Y., Hashimoto T. & Hashimoto N. (1997), Turbulent deposition of aerosol nanoparticles on a wire screen, Aerosol Science & Technology, 27, 471-480.
311
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
C–P–4
Commissioning of HEPA Filter Test Facility - Experiences and Challenges in Nuclear Industry
U.Madhusoodanan, K. Suresh, V. Subramanian
and V. Meenakshisundaram
Radiological Safety Division,
IGCAR, Kalpakkam- 603 102
ABSTRACT : This paper explains the important experiences encountered during the course of commissioning of the HEPA filter test facility and at the various radioactive areas of facilities in the nuclear installations. Since the commissioning of the facility, around 90 fresh filters and 60 online filter banks were tested for the efficiency. This lab successfully carried out the challenging tasks of testing the Deep Bed Off Gas Filters of reprocessing plant and Irradiated fuel pin metallography cell exhaust filter bank. These tests were successfully carried out first time since inception without causing any spread of contamination and personnel exposure which could not be carried out by usual procedure. Iodine filter tests were also carried out after the initial HEPA test. This led to the evolution of a suitable technical procedure to be followed in the nuclear installations.
Key words: HEPA, Aerosol, In-situ testing, Off Gas Filters, Pressure drop
Introduction
HEPA filters, once known as absolute filters, were originally developed as the particulate stage of a chemical, biological, radiological filtration/ adsorber unit for use by the U.S. Armed Services. In the late 1940s the U.S. Atomic Energy Commission adopted them for use for the containment of airborne radioactive particulates in the exhaust ventilation systems of experimental reactors as well as for use in other phases of nuclear research. Nowadays, this technology has emerged to house HEPA filters to deliver clean air to production/sterilized areas as well as for hazardous containment applications. HEPA filter bank (High Efficiency Particulate Air - Filter) is an assembly containing throwaway extended media of dry type filter, with a rigid casing enclosing the full depth of pleats. The filter exhibit minimum efficiency of 99.97% for the test aerosols of size 0.3 m
Prior to installation of filter banks at our laboratories, HEPA filters, fall under the quality assurance standards, are to be evaluated for their performance at our site on random basis (statistical basis). Further, it becomes mandatory to test the filtration system in-situ at regular interval to ensure that it meets the regulatory requirements. Taking into account of huge volume of filtration system employed in IGCAR (taking into account of up-coming active facilities) and to share the work load of BARCF HEPA Filter testing group, a HEPA Filter Testing Laboratory (HFTL) was commissioned by Safety Group at IGCAR in the year 2008 and it is dedicated to test HEPA filters of active facilities of IGCAR.
312
IASTA-2010
Objective
The objective of HEPA filter testing is to ensure a high level of quality i.e., the environment is free from air-borne particulate activity, which is achieved through adoption of a system of procedures [1,2] that reflect the competence of the filter testing facility to the user facility as well as to the independent auditing authorities.
Figure 1. The test rig installed at HEPA Lab, RSD, IGCAR
Test procedure
The HFTL is equipped with digital portable air velocity meter to monitor the flow rate in the exhaust system, differential pressure across the filters. Aerosol Generator (M/s Air Techniques International, USA (Model No.TDA-4B) for the generation of challenge aerosol and the dual particle counter supplied by GRIM Aerosol Technik GmbH&Co, Germany, to count the upstream and down stream concentration simultaneously. The HFTL houses
Figure 2. Graphical representation of quantum of in-situ test
Figure.3 Graphical representation of quantum of rig test
313
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
a test rig for undertaking the testing of individual fresh HEPA filters as per IAEA Technical Series-122. The photograph of the Test Rig is shown in Fig.1. The test rig is constructed and commissioned as per British Standard BS2831.The technical details of test rig is shown in Table 1.The technical specification of the aerosol generator and particle counter is given in Table 2 and Table 3 respectively. The volume of airflow is found out using a digital air velocity meter (Model No 9995) supplied by Trust Science Innovation, USA. The filter efficiency is challenged by generating approved test aerosols Di-Octyl Phthalate (DOP) The performance of the filtration system may be impaired due to leakage paths between the filter gaskets and the frame at various stages as well as deterioration of compaction of filter media. Figs 2&3 show the graphical representations of the tests carried out (in- situ as well as in the Rig).
Table 1. Technical details of the test rig commissioned at HEPA lab
| Upstream length (US) | - | 5m |
| Expander | - | 0.6m |
| Down stream length (DS) | - | 1.3m |
| Expander | - | 0.8m |
| Duct dia. | - | 0.3m |
| Blower | - | 1.3m 3/s |
| Compressor | - | 3HP |
| (double cylinder) | ||
Table 2. Technical specification of aerosol generator
| Output range | 50-8,100cfm |
| Concentration | 100μg/l@810cfm |
| Generator type | 1to6 Laskinozzles |
| Compressed air | 3to18cf @ 20psig |
| Aerosol type | Polydispersed |
Table 3. Technical specification of particle counter
Around 90 fresh HEPA filters of various facilities have been tested in the test rig and 60 in-situ tests have been carried out by HFTL personnel. Many fresh filters showed less than the acceptable efficiency of 99.97% due to completion of the storage life time (36 months after procurement). The
adhesives used in the filter media had worn out as seen by naked eye. This was cross verified by the pressure drop across the filter before the injection of the aerosol itself. Some of the new filters after procurement and not completing the storage time also showed
Figure 4. The schematic diagram of the fresh filter showing the faults in the fresh filters as procured
314
IASTA-2010
lesser efficiency due to the fact that the screws on the frame were missing and the leakage of air by laminar flow through these voids. This is shown as a schematic diagram (fig.4)
Deep Bed Off Gas Filter( DBOG Filter) test
The deep bed filter of the reprocessing plants are very important from the point of view of filtering of off gas from the processing system to the stacks of the plant. This filter system is very complicated from the point of maintaining negative pressure and filtering the air twice before sending it to stack of the reprocessing plant. Testing this filter is very difficult as it may contaminate the counting system while counting the upstream counts. This problem was over come by suitably calibrating the aerosol generator to produce aerosol particles at particular number concentration by using same air velocity of the DBOG blower. This generator was taken to the DBOG system and downstream counts were taken after injecting the aerosol at the upstream. After the run was over the aerosol concentration injected was cross checked again at the rig facility. Thus the efficiency of the DBOG filter was calculated for the first time to make sure the exact efficiency of the current filter. In normal cases such filters will not be tested and will be replaced on a time bound basis.
Iodine Filter test
4 in-situ iodine tests have also been completed during this year in the reactor containment building. Before procuring charcoal filter FBTR initiates the evaluation of activated charcoal. The sample of activated charcoal sent by the supplier is evaluated for the following parameters: Surface area, Hardness number, Moisture content, CCl4 activity, Iodine number, Ignition temperature, Apparent density, Impregnants (KI & KOH) and Mesh size [3,4]. During these tests the air activity as well as contamination checks were carried out and no case of contamination and air activity was observed.
Quality Assurance: The objectives are met by the involvement of all the staffs who are responsible for their work. The quality management system of HFTL adopts 4 levels of documents viz. Level 1: Quality Manual, Level 2: Quality Procedure, Level 3: Work Instruction and Level 4: Quality forms. A clear documentation procedure is adopted for the receipt of the requests from various laboratories and reports of the filter testing by the lab.
Conclusion
A HEPA filter laboratory has been successfully commissioned and the documentation for the testing procedures has been established. HFTL is committed to provide services in the field of testing HEPA filter banks and Iodine Filter banks in various facilities with high credibility and integrity, which consistently satisfy the needs and expectations of our centre.
References
1.Technical reports series No.122
2.IGC/SG/RSD/RSS/92628/TS/3002/REV-A
3.Technical reports series No.243
4.IGC/SG/RSD/RSS/92628/TS/3006/REV-A
315
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
C–P–5
Study on Atmospheric and Radioactive Aerosols Around Bangalore
C. Ningappa1, J. Sannappa2 and B. Nagappa3
1Vidya Vikas Institute of Engineering and Technology,Mysore – 570 010, India
2Department of Physics,Yuvaraja’s College, University of Mysore, Mysore-570 005,
3Karnataka State Pollution Board, Bangalore – 560 001
Email: sannappaj@yahoo.co.in, sannappaj@gmail.com
The increasing environmental pollution mainly due to industrialization and urbanization has given rise to concern about the accumulation of toxic waste in the atmosphere.
The vehicular exhaust is a complex mixture comprising of hazardous particle and vapors. According to the World Health Organization, New Delhi is one of the top ten most polluted cities in the world. There are seven primary air pollutants, which are considered as health hazard when their concentration exceeds the national ambient air quality standards. These are Suspended Particulate Matter (SPM), carbon monoxide, nitrogen dioxide, ozone and other photochemical oxidants, lead and sulphur dioxide. Other pollutants include toxic gases and heavy metals [1]. In addition to these, radon, thoron and their decay products exist in our environment can cause the occurrence of lung cancer [2].
Aerosols are injected into the atmosphere in varying proportions and reside in the atmosphere for few hours to few days depending on the size of the particles. They play a major role in modifying the weather by altering the energy budget of the earth atmosphere system through scattering and absorbing the solar radiation as well as by altering the cloud parameters such as cloud droplet size and cloud lifetime. The large surface area of these particles, provide sites for chemical reactions to take place. These reactions lead to the formation of large amounts of reactive chlorine and ultimately to the destruction of ozone in the atmosphere.
Aerosols can have a tremendous effect on the earth and its atmosphere. Aerosols that are found in the upper atmosphere do not absorb solar radiation like greenhouse gases. Instead they scatter sunlight and reflect the radiation back into space. This reflection causes the earth to receive less energy than normal, cosequently helping to lower the earth’s temperature. Naturally occuring aerosols (troposphere) cool the surface from 2 to 3°C [3]. If enough aerosols were to accumalate in the atmosphere, it is possible that they could counter the temperature increase caused by the greenhouse effect. Radiative properties of aerosols can give rise to changes in the earth atmosphere system. They effect the satellite remote sensing and air and water quality. Water-soluble aerosols act as condensation nuclei and initiate the cloud formation process. In these ways aerosols particulate in changes of thermal equillibrium and are therefore major contributes to thermodynamic exchange processes in the atmosphere [4]. Due to their absorptive nature, aerosols such as smoke clouds also warm the atmosphere and contribute to changes in atmospheric circulation [5]. Therefore long term monitoring of aerosol properties will be
316
IASTA-2010
useful in modelling of aerosol parameters which are very important in radiation budget calculation and modelling of climatic impacts.
The above facts and the research work done earlier indicate that there is a great need to analyze the level of aerosols around Bangalore. The objective of the present study is to analyze the different types of aerosols in ambient air in a few places of Bangalore city with the help of important available data from Karnataka State Pollution Control board and measurement of concentrations of radon and its progeny. Radon and its progeny concentrations around Bangalore were measured using Solid State Nuclear Track Detectors (SSNTD) [6].
In Bangalore, the ambient air pollutants have been carried out by pollution control board under National Ambient Air Monitoring Programme (NAMP). The Cental Pollution Control Board (CPCB) conducts an annual exercise of ranking based on the RSPM data collected from over 3000 stations located in 115 cities across the country. In Karnataka, three stations in Bangalore and two in Mysore record data. In Bangalore, the stations are located in Amco Batteries premises on Mysore road, Graphite India in Whitefield and Ananda Rao circle. The monitoring is being carried out at a frequency of twice a week for 24 hours for Suspended Particulate Matter (SPM), sulphur dioxide (SO2), oxides of nitrogen (Nox) and Respirable Suspended Particulate Matter (RSPM). Under this programme 5195 samples were collected and analysed. During the year 2003 – 2004 the annual arithmetic mean of the concentration of the pollutants are shown in Table-1.
Table -1 indicates the arithmetic mean values of concentration of pollutants in Bangalore city, which was being monitored at three stations under NAMP. The higher concentration of pollutants in industrial areas is due to factories like wood, food processing, cloth, metal reformers, painting and small-scale industries. The pollutants at Ananda Rao circle are higher than AMCO batteries and Graphite India, Whitefield. The higher concentration in Ananda Rao circle is due to the maximum number of vehicles that pass by the circle [7].
Table 1. Ambient air quality of Bangalore city among three stations
| Si.No | Station | SO2 gm-3 | Nox gm-3 | SPM gm-3 | RSP Mgm-3 | |
| 1 | Ananda Rao circle | 7.8 | 46 | 195.7 | 80.9 | |
| 2 | AMCO Batteries, Mysore road | 6.7 | 28.4 | 181.4 | 78.1 | |
| 3 | Graphite India, Whitefield | 16 | 37.5 | 139.5 | 62.7 | |
The results of concentrations of radon and its daughter products were measured in different regions of Bangalore city are summarized in Table -2. Higher concentration of radon and its progeny were observed in HSR layout. This area is attributed by granite. Granites containing higher concentration of radium. Higher the concentration of radium, higher will be the concentration of radon [8]. Slightly less concentration of radon and its progeny were observed in JP Park, Graphite India, Whitefield and Mathikere compared to HSR layout. In these regions grey rocks were observed. These rocks contain less concentration of radium compared to granites. Minimum concentration of radon and its progeny were observed in Vijayanagara. Dolerites are observed in this area. Dolerites containing less concentration of radium.
The inhalation equivalent effective dose rate due to radon and its progeny in the study area varies from 0.17 to 0.57 mSv.y-1 with a median of 0.35 mSv.y-1.
317
AEROSOLS & CLOUDS : CLIMATE CHANGE PERSPECTIVES
Table 2. Concentration of radon, its progeny and effective dose due to these
| SI.No | Station | 222Rn Conc | 222Rn Prog. Conc. | Dose |
| (Bq.m-3) | (mWL) | (mSv.y-1) | ||
| 1 | Ananda Rao circle | 26 | 0.36 | 0.36 |
| 2 | AMCO Batteries, Mysore road | 23 | 0.38 | 0.32 |
| 3 | Graphite India, Whitefield | 31 | 0.49 | 0.40 |
| 4 | Vijayanagara | 12 | 0.34 | 0.17 |
| 5 | Rajaji Nagar | 24 | 0.40 | 0.33 |
| 6 | Yashwanthapuram | 21 | 0.49 | 0.29 |
| 7 | Mathikere | 30 | 0.52 | 0.41 |
| 8 | HSR Layout | 41 | 0.76 | 0.57 |
| 9 | JP Park | 34 | 0.69 | 0.47 |
| Minimum | 12 | 0.34 | 0.17 | |
| Maximum | 41 | 0.76 | 0.57 | |
| Geometrical Mean | 25.6 | 0.47 | 0.35 | |
The higher concentration of pollutants in industrial areas is mainly due to factories like wood, food processing, cloth, metal reformers, painting and small-scale industries. The higher concentration is also due to the maximum number of vehicles in the city. The concentration of radon is mainly depends on the activity of radionuclides present in soil and rocks. The results show that the impacts of radiation hazard on the public due to radon and its progeny. As per the ICRP recommendation, it becomes necessary to take remedial steps for the reduction of radon and its progeny in dwelling places, if the level is found to be more than 200 Bq.m-3.
References
1.D K Pandey; Effects of Air Pollutants on Health, Employment News; 26 (33), 48 -49, 2001.
2.UNSCEAR, United Nations Scientific Committee on the Effects of Atomic Radiation. Sources, Effects and Risks of Ionizing Radiation, Report to the General Assembly, United Nations, New York, 2000
3.A Jayaraman ; Atmospheric Aerosols and Climate Change, Physical Research Laboratory, Ahamadabad, 1998, 2001.
4.G A D’ Almeda, Kocpkep and E D Shelton; Atmospheric Aerosols- Global Climatology and Radiative Charecteristics; A Deepak publishing and Hampten V A, 1991.
5.Kaufonam and T Nakajima; Group report, Connection between Aerosol Properties and forcing of Climate; 1995.
6.Eappen K P, Ramachandran T V, Shaik A N and Mayya Y S. Calibration factors for SSNTD based radon / thoron dosimeters, Radiat. Prot. and Environ., 24 410 2001
7.Karnataka State Pollution Control Board, Bangalore; Annual report, 2003-2004, 12-15.
| 8. | Bachli, R, Burkart W Influence of subsoil geology and construction technique on indoors air | 222Rn |
| levels in 80 houses of the central Swiss Alps. Health Phys. 56 423 1989. | ||
318